AG Thomas Winkler
Focus of research: AG Thomas Winkler
The group of Professor Thomas Winkler is interested in the regulation of processes generating immune receptor diversity and autoimmunity.
Prof. Dr. Thomas Winkler
Professur für Genetik (Prof. Dr. Winkler)
- Telefon: +49 9131 85-29136
- E-Mail: thomas.winkler@fau.de

During the development of B lymphocytes, the genes encoding immunoglobulins are compiled in a process of genetic recombination, that is responsible for the enormous variability of the antibody repertoire. We are interested in the regulation of this process, which is called VDJ-recombination. Although its mechanism is well understood, its regulation is still unclear. For example, recombination of V-, D- and J-segments of all seven antigen-receptor gene clusters (3 immunoglobulin-gene cluster & 4 T-cell receptor gene cluster) occurs through the same enzyme machinery, but its expression is tightly regulated. Antibody genes are exclusively expressed in B cells, while T cell receptor genes are only recombined in T lymphocytes. In addition, recombination of gene segments for the light and heavy receptor chains is coordinated in a timely manner (Fig. 1), leading to each B lymphocyte only expressing one combination of heavy and light chain (a process termed allelic exclusion).
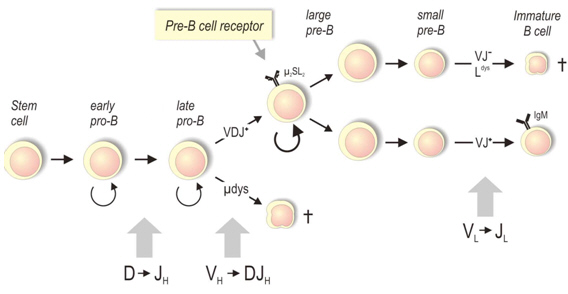
The focus of our research work lies in the approachability of the Ig heavy chain gene cluster for the VDJ recombinase. We investigate epigenetic mechanisms of gene regulation through methods of chromatin analysis (ChIP-Seq, ChIP-on-Chip) and try to identify regulatory sequences within the IgH-gene locus.
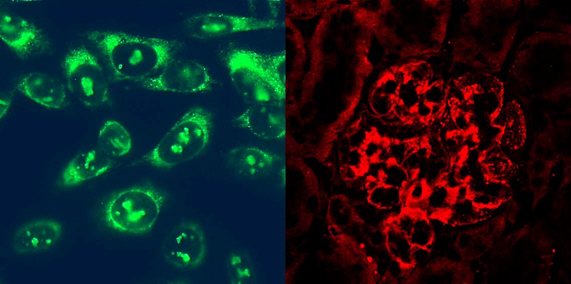
Autoantibodies against DNA (ANAs) are characteristic for the autoimmune disease systemic lupus erythematodes (SLE). In our group, we investigate both the mechanisms of immunologic dysregulation that are causal for the emergence of ANAs, as well as their role in SLE pathology, especially for glomerulonephritis. In our projects, we work on murine models of SLE. Herein, we utilize spontaneous and genetically modified disease models, that we established through gene targeting.
Currently, we follow the hypothesis that such autoantibodies arise due to dysregulations during a process called germinal centre reaction. To adress this hypothesis, we use a mouse model overexpressing a patient-derived autoantibody.
Infection with the human cytomegaly virus (HCMV, HHV5) usually occur without onset of symptoms, with the virus being prevalent in 50-90% of the adult human population. Severe disease occurs in patients with a compromised immune system, as for example after an organ graft. Additionally, HCMV is an important congenically transferred pathogen, that can lead to severe prenatal manifestations.
In order to better understand the humoral immune response against this virus, our group cooperates with the group of Prof. Michael Mach from the Institute of Clinical Virology (University Hospital Erlangen). We hope to develop novel therapeutic concepts on the foundation of our findings.
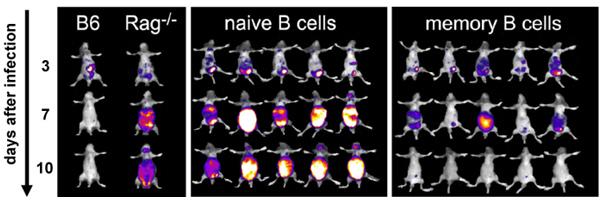
We investigate both the human cytomegaly virus (HCMV) as well as its murine relative (murine cytomegaly virus, MCMV). Utilizing mice, we model MCMV infection in the context of clinically relevant immunosupression after bone-marrow transplantation. Novel therapeutic concepts utilizing memory B cells or monoclonal antibodies are tested in these mouse models. In a cooperation with the University Hospital Erlangen Clinic V, we want to translate these concepts into a clinical setting.
Thomas Winkler on Google Scholar
5 Key Publications
- A.M. Hahn, L. Vogg, S. Brey, A. Schneider, S. Schäfer, R. Palmisano, A. Pavlova, L. Sandrock, L. Tan, A.S. Fichtner, I Prinz, S. Ravens and T.H. Winkler. A monoclonal Trd chain supports the development of the complete set of functional γδ T cell lineages. Cell. Rep. 2023 Mar 28;42(3):112253. doi: 10.1016/j.celrep.2023.112253. Epub 2023 Mar 14. PMID: 36920908
- Irrgang P., J. Gerling, K. Kocher, D. Lapuente, P. Steininger, K. Habenicht, M. Wytopil, S. Beileke, S. Schäfer, J. Zhong, G. Ssebyatika, T. Krey, V. Falcone, C. Schülein, A.S. Peter, K. Nganou-Makamdop, H. Hengel, J. Held, C. Bogdan, K. Überla, K. Schober, T.H. Winkler and M. Tenbusch. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023 Jan 27;8(79):eade2798. doi: 10.1126/sciimmunol.ade2798. Epub 2023 Jan 27. PMID: 36548397
- Mackensen A., F. Müller, D. Mougiakakos, S. Böltz, A. Wilhelm, M. Aigner, S. Völkl, D. Simon, A. Kleyer, L. Munioz, S. Kretschmann, S. Kharbiutli, R. Gary, H. Reimann, W. Rösler, S. Uderhardt, H. Bang, M. Herrmann, A.B. Ekici, C. Buettner, K.M. Hebenicht, T.H. Winkler, G. Krönke and G. Schett. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. med. 2022 Oct;28(10):2124-2132. doi: 10.1038/s41591-022-02017-5. Epub 2022 Sep 15. PMID: 36109639
- Weisenburger T., B. von Neubeck, A. Schneider, N. Ebert, D. Schreyer, A. Acs and T.H. Winkler.Epistatic Interactions Between Mutations of Deoxyribonuclease 1-Like 3 and the Inhibitory Fc Gamma Receptor IIB Result in Very Early and Massive Autoantibodies Against Double-Stranded DNA. Front. Immunol. 2018 Jul 5;9:1551. doi: 10.3389/fimmu.2018.01551. eCollection 2018. PMID: 30026744
- Seefried M., N. Hundhausen, I. Kroeger, M. Büttner-Herold, P. Hoffmann, M. Edinger, E. Ullrich, F. Berberich-Siebelt, W.J. Britt, M. Mach, and T.H. Winkler. Murine cytomegalovirus reactivation concomitant with acute graft-versus-host disease is controlled by antibodies. JCI Insight. 2023 Mar 8;8(5):e149648. doi: 10.1172/jci.insight.149648.
Click here for the full list of publications
