AG Lars Nitschke
Focus of research: AG Lars Nitschke
The group of Professor Lars Nitschke is interested in proteins of the siglec family that, among other functions, modulate B cell receptor signalling.
Prof. Dr. Lars Nitschke
Professur für Genetik (Prof. Dr. Nitschke)
- Telefon: +49 9131 85-28453
- E-Mail: lars.nitschke@fau.de

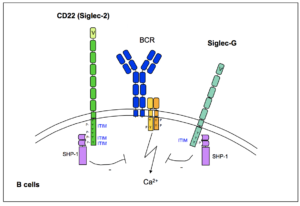
Fig. 1: The functions of CD22 and Siglec-G in the modulation of BCR-mediated signalling.
B lymphocytes (or B cells) are cells of the adaptive immune system, which induce an antibody-mediated humoral immune response.
We are interested in the signals, that regulate development and functionality of B cells. CD22 (Siglec-2) and Siglec-G are B cell transmembrane proteins that are associated with the B cell receptor (BCR) and interfere with its signalling cascade (Fig. 1). Both carry inhibitory ITIM motifs, which are phosphorylated after stimulation of the BCR. After phosphorylation, the ITIM motif recruits phosphatases such as SHP-1, which negatively regulate signalling.
We generated CD22-/- and Siglec-G-/- mice and could show that these mice exhibit greatly increased BCR signalling, as measured through Ca2+ influx into the B cell. CD22 inhibits BCR signalling in conventional B cells, while Siglec-G inhibits BCR signalling in the subpopulation of B1 cells, which are B cells with specific functions.
Inhibitory proteins like Siglecs are important to control B cell activation, because uncontrolled activation of B cells could lead to the emergence of autoimmune disease or to the development of B cell lymphomas or leukemias. Both Siglec-G-/- , as well as CD22-/- x Siglec-G-/- double deficient mice develop an autoimmune disease which resembles systemic lupus erythematosus (SLE), characterized by autoantibodies and kidney pathology. As the inhibitory Siglec proteins are involved in preventing theses diseases, new treatment options targeting these proteins could be developed. This project is funded by the TRR130.
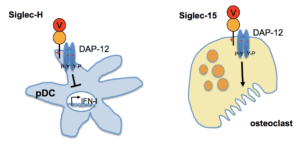
Siglec-H is expressed in a very restricted fashion on plasmacytoid dendritic cells (pDCs), while Siglec-15 is expressed exclusively on osteoclasts (Fig.2). Siglec-H inhibits the type1 interferon (IFN-I) production in pDCs. IFN-I is important for anti-viral responses, but is also elevated in SLE patients. We could show, that Siglec-H-/- mice develop a very strong SLE-like autoimmune disease several weeks after a virus (CMV) infection. This shows that viral infections can trigger an autoimmune disease when IFN-I is not well controlled. Siglec-15 is crucial for osteoclast maturation and plays a role in bone erosion in arthritis, as we could show with Siglec-15-/- mice that we generated. This implies Siglec-15 as a potential new target in arthritis and also in osteoporosis. This project is funded by the CRC 1181.
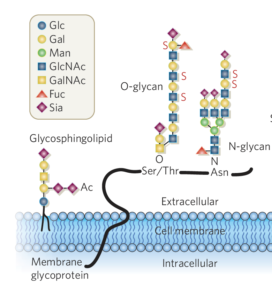
Sialic acids (Sia) are abundant carbohydrate structures on glycoproteins of cell membranes. On these glycoproteins Sia are the terminally expressed carbohydrates (Fig.3). Sia serve as ligands for Siglec proteins, regulating their inhibitory functions.
We generated mice lacking Sia either on B cells or T cells. Surprisingly, B cells and T cells without Sia cannot survive in immunological organs such as the spleen or lymph nodes.
This points to a more general role of Sia than just serving as Siglec ligands on lymphocytes. These functions will be addressed in our group in the future. This project is funded by the FOR 2953.
Lars Nitschke on Google Scholar
5 Key Publications
- Müller J, Nitschke L. (2014) The role of CD22 and Siglec-G in B-cell tolerance and autoimmune disease. Nat Rev Rheumatol. 10:422-8.
- Schmitt H, Sell S, Koch J, Seefried M, Sonnewald S, Daniel C, Winkler TH, Nitschke L. (2016) Siglec-H protects from virus-triggered severe systemic autoimmunity. J Exp Med. 213:1627-44
- Müller J, Obermeier I, Wöhner M, Brandl C, Mrotzek S, Angermüller S, Maity PC, Reth M, Nitschke L. (2013) CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci U S A. 110:12402-7
- Ackermann J, Radtke D, Maurberger, A, Winkler TH, Nitschke L (2011) Grb2 regulates Bcell maturation, B cell memory responses and inhibits B cell Ca2+ signalling. EMBO J. 30:1621-1633
- Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. (2007) Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 8:695-704.
Click here for the full list of publications
Research of the Nitschke group is funded in the context of the following projects:
- DFG TRR130/C04: Speaker of the CRC (2017-2022)
- DFG TRR130/P04: The role of Siglec proteins in B cell activation and autoimmunity (2017-2022)
- DFG RTG2599: The role of SIGLEC10 and DNAse1 mutations in SLE (2021-2025)
- DFG FOR2953/P02: The importance of sialoglycans for lymphocyte development (2022-2025)
- DFG CRC1181/B06: The role of SIGLEC-H and SIGLEC-15 in induction and resolution of inflammation (2019-2023)
