AG Anja Lux
Focus of research: AG Anja Lux
The group of Prof. Dr. Anja Lux is interested in effector mechanisms of B cell depleting antibodies and Fc receptor dependent activation of immune cells.
Prof. Anja Lux
Chair of Genetics
- Telefon: +49 9131 85-25048
- E-Mail: anja.lux@fau.de

Therapeutic monoclonal antibodies (mAbs) directed against the CD20 surface marker of B cells e.g. Rituximab or Ofatumumab are widely used to treat malignant as well as inflammatory disorders in humans as they cause efficient depletion of CD20 expressing B cell populations. The effector mechanisms underlying CD20 mAb activity are discussed to involve the mononuclear phagocytic cells, NK cells or the complement system. However, CD20 mAb activity has predominantly been studied in mouse models or in vitro and the effector mechanisms in humans in vivo are still unclear.
One focus of our research is to fill this gap in knowledge by studying CD20 mAb induced B cell depletion in human immune system mice. These so-called humanized mice are characterized by the long-term presence of a wide spectrum of human hematopoietic cells including developmental precursor populations and therefore allow for analysis of human immunoglobulin G (IgG) in a human immune system in vivo. We thereby aim to identify immune effector cells involved in B cell depletion and characterize potential organ niches with reduced mAb activity (Figure 1).
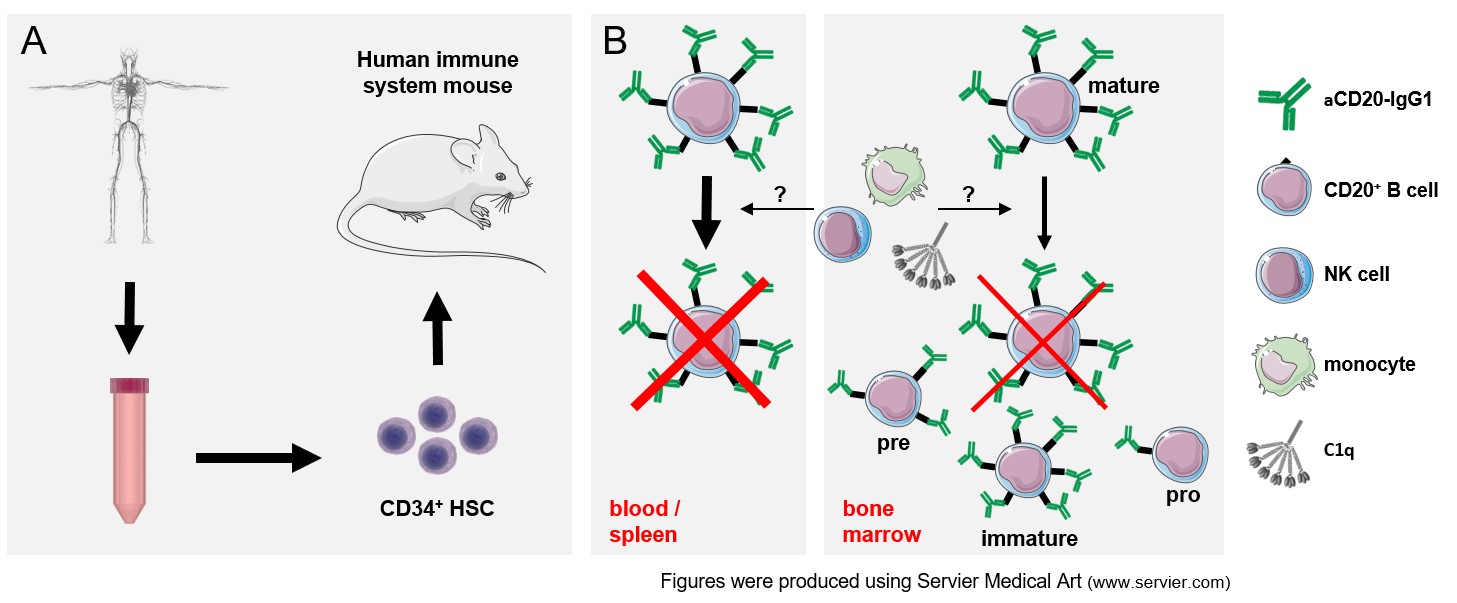
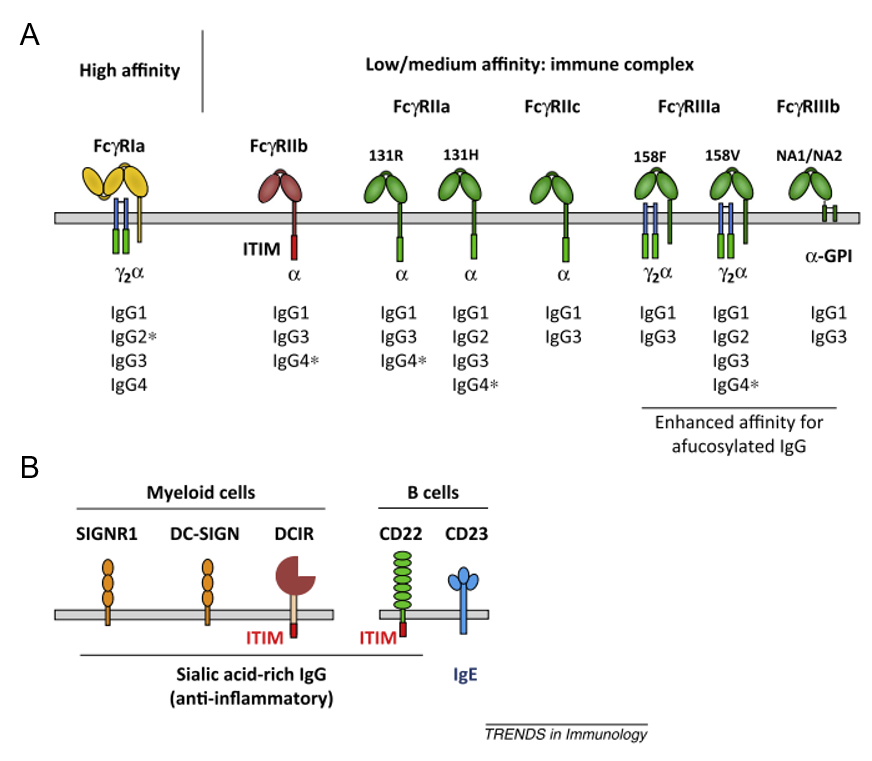
Most types of human immune cells express IgG binding Fc receptors (FcγR). By interacting with IgG, these FcγR are crucially involved in bridging innate and adaptive immune responses. FcγR come, however, in different flavours: They can initiate or dampen immune cell activation and they might be able to interact with monomeric IgG (high-affine CD64) or only with IgG in larger antibody-antigen immune complexes (low to medium affine CD32 and CD16). Most importantly, many immune cell subsets co-express different kinds of FcγRs to various degrees and the situation becomes even more complex due to the presence of so-called type II Fc receptors only recently recognized to interact with IgG.
To date, the individual contribution of FcR on different immune cell types and a potential cell-type specific receptor activity are largely unknown. Therefore, we currently study IgG immune complex binding to FcR and its effects on immune cell function with a focus on the role of immune complex size, IgG subclass and glycosylation and FcγR polymorphisms. Given that FcR are transmembrane- or membrane-anchored surface receptors, we further investigate if immune cell type specific plasma membrane environments affect FcR function.
The primary function of innate and adaptive immune cells is to protect us from invading pathogens. However, the same mechanisms involved in protective immune responses are also responsible for the pathogenesis of autoimmune diseases if the careful balance of immune cell activation and inhibition is disturbed. Surprisingly, the causative immune dysregulation may precede clinical manifestation of disease in humans by many years while the reasons for this phenomenon are still unknown. In a mouse model of inflammatory arthritis, we are currently investigating changes in innate immune cell function and their contribution to the switch from asymptomatic to symptomatic disease.
Autoimmune diseases are chronic inflammatory diseases caused by immune reactions against cells and tissues. In consequence, patients suffering from rheumatoid arthritis or autoimmune skin blistering diseases present with destructive inflammation of the joints or skin, respectively. Despite immense recent advances in understanding and therapy of autoimmunity, our knowledge on its pathogenesis is still incomplete. While classical mouse models are essential to elucidate the underlying mechanisms and develop therapeutic strategies, differences in comparison to the human immune system hamper transfer of knowledge. In current projects, we thus employ humanized mice, i.e. mice reconstituted with a human immune system upon hematopoietic stem cell transfer, to establish in vivo models for inflammatory arthritis and skin blistering disease. We thereby aim to elucidate disease pathogenesis and test for clinical efficacy of novel therapeutics in a human immune system background.
Anja Lux on Google Scholar
5 Key Publications
- Kara S, Amon L, Lühr JJ, Nimmerjahn F, Dudziak D, Lux A. Impact of plasma membrane domains on IgG Fc receptor function. Frontiers in Immunology. 2020.
- Kao D, Danzer H, Collin M, Groß A, Eichler J, Stambuk J, Lauc G, Lux A, Nimmerjahn F. A Monosaccharide Residue Is Sufficient to Maintain Mouse and Human IgG Subclass Activity and Directs IgG Effector Functions to Cellular Fc Receptors. Cell Rep. 2017. 47(12):2070-2079.
- Lux A, Seeling M, Baerenwaldt A, Lehmann B, Schwab I, Repp R, Meidenbauer N, Mackensen A, Hartmann A, Heidkamp G, Dudziak D, Nimmerjahn F. A humanized mouse identifies the bone marrow as a niche with low therapeutic IgG activity. Cell Rep. 2014. 7(1):236-48
- Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol. 2013. 190(8):4315-23
- Lux A, Nimmerjahn F. Of mice and men: the need for humanized mouse models to study human IgG activity in vivo. J Clin Immunol. 2013. 33 Suppl 1:S4-8
Click here for the full list of publications
Research of the Lux group is funded in the context of the following projects:
- DFG FOR2886/B2: Die IgG-Glykosylierung in der Regulation der rheumatoiden Arthritis (2019-2021)
- DFG GRK2504/C5: Inducing long-lasting HIV Env-specific antibody responses by intrastructural help (2019-2024)
- DFG GRK2599/P4: Regulation of immune complex-mediated DC-dependent immune responses by DCIR (2020-2025)
- DFG SFB1526/B4: Kinase cascades in neutrophils as therapeutic target in PDS (2022-2025)
